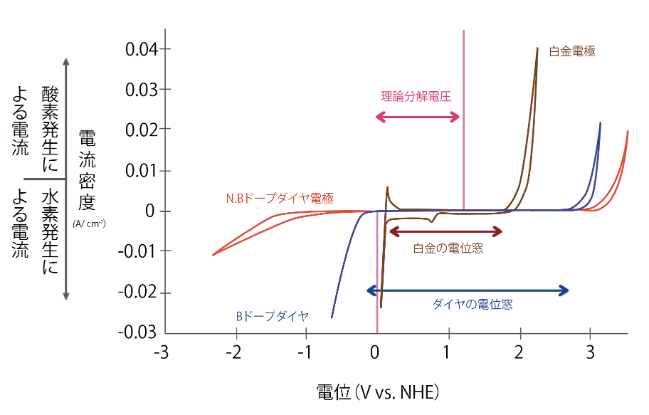
Lineup of diamond electrodes
We not only manufacture and sell diamond electrodes (BDD: Boron Doped Diamond) alone, but also offer two types of product lineup that apply BDD.
1)BDD spiral module
Introducing the BDD spiral module for ozone water generation using an ion exchange membrane. This product was developed in collaboration with Professor Kitaori of Tokyo National College of Technology. The electrolytic part (membrane-electrode assembly) is shown in [Figs. 4 and 5].
It has a structure in which an ion exchange membrane and a cathode ray are wound around a rod-shaped BDD anode, and has the following features.
1) This module features an one-chamber cell structure that does not separate hydrogen, ozone, and oxygen.
2) Since it has an one-chamber cell structure, it is electrolyzed in the neutral region, so hardness components such as Ca and Mg do not easily adhere, and tap water can be used as a raw material.
3) Using tap water as a raw material, ozone water of 1 to 2 mg dm-3 can be stabilized for 300 hours under the conditions shown below.
When tap water is used as a raw material and electrolyzed at a constant voltage of 15 V (repeated operation of 8 minutes ON + 2 minutes OFF), a current of about 0.5 A is stably applied, and 1 to 2 mg dm-3 ozone water is applied for 300 hours. You can get stable over.
The current efficiency is reduced to about one-third compared to the case of pure water raw material, but this is due to the decrease in ozone current efficiency due to changes in the current distribution and impurities such as chloride ions, effective chlorine and hardness components. It is presumed that the generated ozone is decomposed due to the presence and reaction with the alkaline component generated at the cathode.